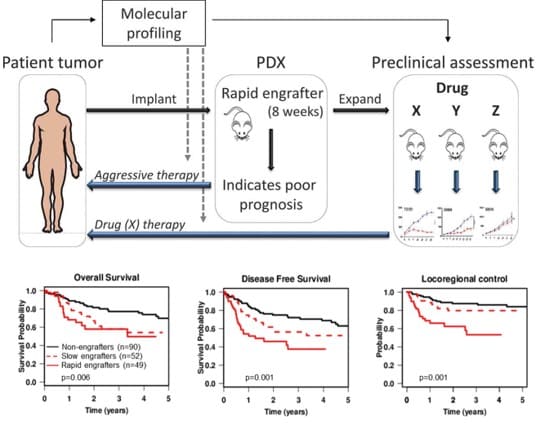
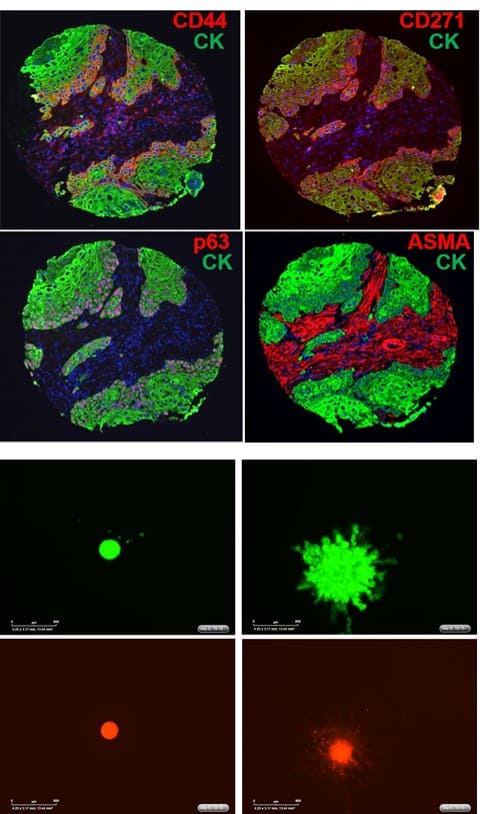
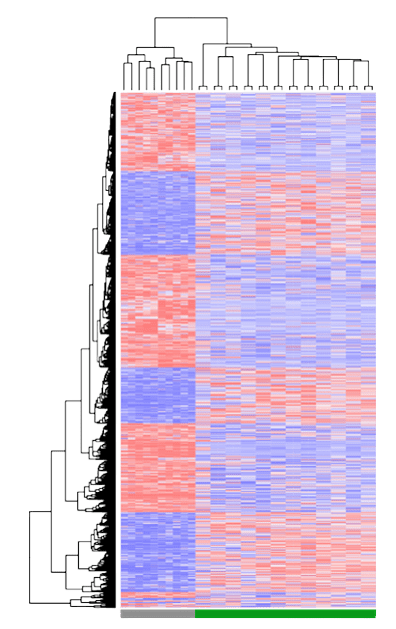
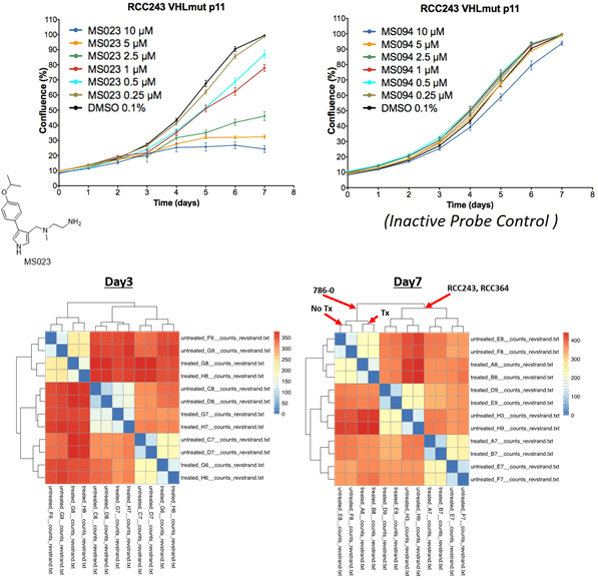
Dr. Ailles got her B.Sc. in Biology at Carleton University
(Ottawa), an M.Sc. in Pathology at Queen’s University
(Kingston), and her Ph.D. in Genetics at the University of
British Columbia (Hogge Lab, Terry Fox Labs). She then did a
post-doc at the Candiolo Cancer Institute (Naldini Lab) in
Turin, Italy, and a second post-doc at Stanford University
(Weissman Lab). She came to Toronto to join the research
faculty at the Princess Margaret Cancer Centre in 2008.
Throughout her research career her interests have been focused
on stem cell and cancer stem cell biology, and the use of
patient-derived models to study cancer.